
In balanced nuclear equations, the sums of the subscripts on each sides of the equation are the same, as are the sums of the superscripts. Using the data given, we can write the following initial equation: n 0 1 + P 94 239 u → A 79 204 u + P 15 31 + ? n 0 1 Write the balanced nuclear equation for the process and determine the number of neutrons given off as part of the reaction. Plutonium-239 can absorb a neutron and undergo a fission reaction to produce an atom of gold-204 and an atom of phosphorus-31.
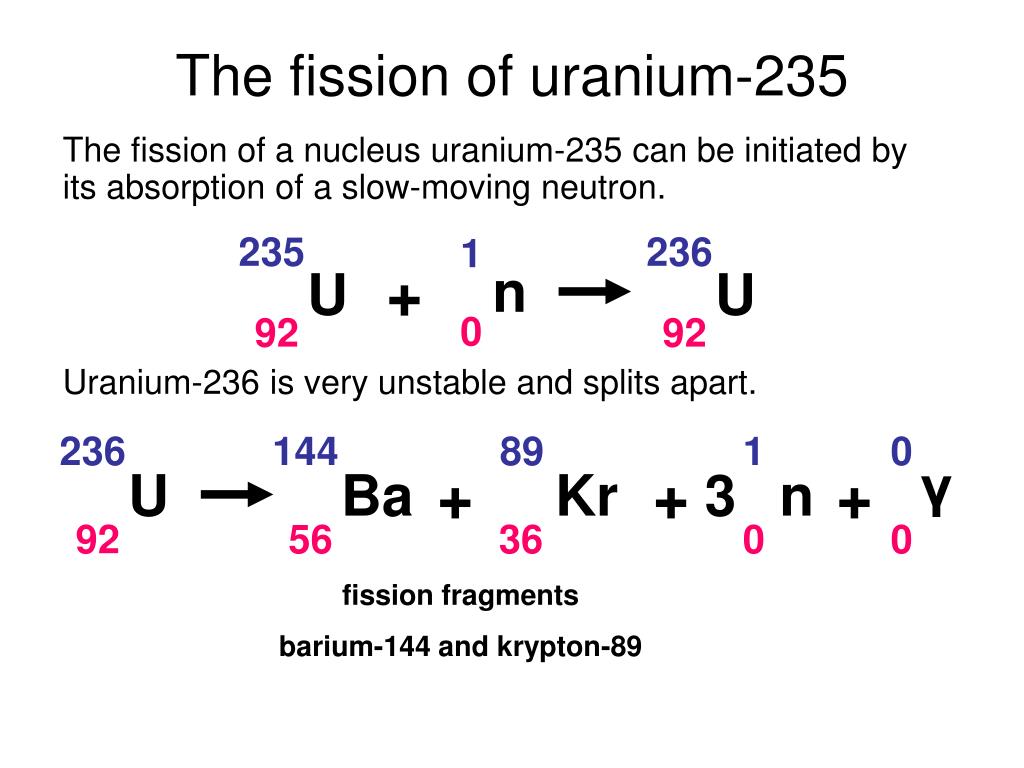
Thus, by the careful addition of extra neutrons into a sample of uranium, we can control the fission process and obtain energy that can be used for other purposes. The overall nuclear equation, with energy included as a product, is then as follows: 235U + 1n → 139Ba + 94Kr + 3 1n + energy The reaction can be controlled because the fission of uranium-235 (and a few other isotopes, such as plutonium-239) can be artificially initiated by injecting a neutron into a uranium nucleus. involves the controlled harvesting of energy from fission reactions. Nuclear energy The controlled harvesting of energy from fission reactions. If this energy could be properly harvested, it would be a significant source of energy for our society. Nuclear reactions give off billions of kilojoules per mole. Compare it to combustion reactions of hydrocarbons, which give off about 650 kJ/mol of energy for every CH 2 unit in the hydrocarbon-on the order of hundreds of kilojoules per mole. This is an extraordinary amount of energy. That is, 16.5 billion kJ of energy are given off every time 1 mol of uranium-235 undergoes this nuclear reaction. In the course of the uranium nuclear chemical reaction, the mass difference is converted to energy, which is given off by the reaction: E = (−0.0001834 kg)(3.00 × 10 8 m/s) 2 = −1.65 × 10 13 J = −1.65 × 10 10 kJ Where c is the speed of light, or 3.00 × 10 8 m/s. Where did this mass go?Īccording to Albert Einstein’s theory of relativity, energy ( E) and mass ( m) are related by the following equation: E = mc 2 If we compare the mass of the reactant (235.0439) to the masses of the products (sum = 234.8605), we notice a mass difference of −0.1834 g, or −0.0001834 kg. Consider the following nuclear reaction, in which the molar mass of each species is indicated to four decimal places: U 235 235.0439 → B 139 a 138.9088 + K 94 r 93.9343 + 2 n 1 2 × 1.0087 Where does this energy come from? If we could precisely measure the masses of the reactants and the products of a nuclear reaction, we would notice that the amount of mass drops slightly in the conversion from reactants to products. Nuclear changes occur with a simultaneous release of energy. Describe the difference between fission and fusion.Explain where nuclear energy comes from.


 0 kommentar(er)
0 kommentar(er)
